The term “triple point” was coined in 1873 by James Thomson, brother of Lord Kelvin.
The triple point occurs where the solid, liquid, and gas transition curves meet.
The triple point is the only condition in which all three phases can coexist, and is unique for every material.
Triple Point of Water
The triple point of water is defined as the temperature and pressure at which the three phases liquid water, solid ice and water vapour can coexist in a stable equilibrium.
The triple point of water is used to define the Kelvin(K), the base unit of thermodynamic temperature in the International System of Units (SI).
The triple point of water is 273.16 K (0.01∘ C or 32.018∘ F).
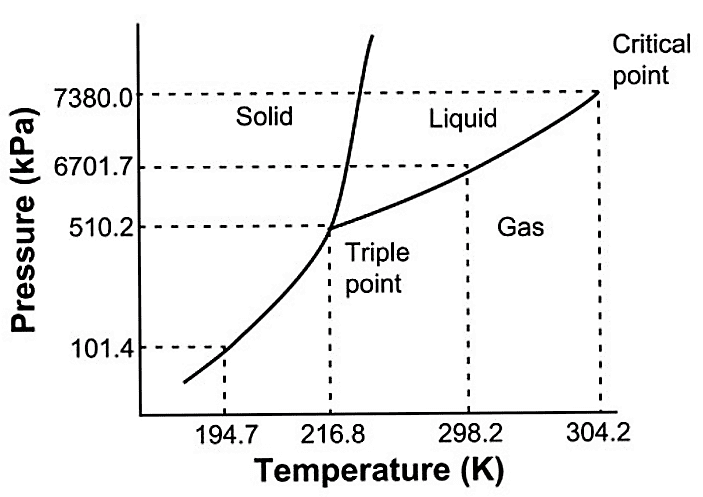
It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporization curve meet.
At triple point of water, both solid (ice) and vapour state are present so we can say that boiling point and freezing point become same.
The single combination of pressure and temperature at which liquid water, solid ice, and water vapour can coexist in a stable equilibrium occurs at approximately 273.1575 K.